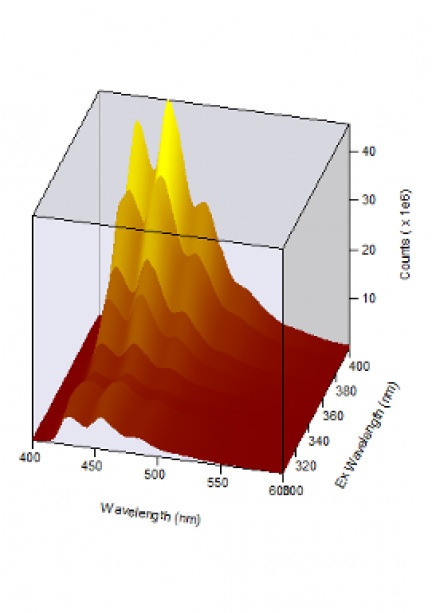
Optical spectroscopy is a branch of science that studies the interaction of light with matter. Its aim is to obtain an optical spectrum, i.e. the dependence of the intensity of radiation absorbed, reflected, emitted or scattered by a substance on the wavelength, from which the properties of the material under study are then derived.
If the substance under study absorbs part of the incident optical radiation, the energy delivered can cause the system to transition to higher energy levels. During transitions to lower levels, the system may emit optical radiation, the detection of which yields an emission (luminescence, fluorescence, phosphorescence) spectrum.
If some of the optical radiation passes through the sample, then the transmittance of the sample is determined as the ratio of the intensity of the radiation that passed through the sample to the intensity of the radiation incident on the sample. From the transmittance, the absorbance of the material is then easily expressed. If we plot the absorbance (or absorption coefficient) versus wavelength, we obtain the absorption spectrum. If only the reflection of the radiation from the surface of the sample is measured, this is the reflectance spectrum.
In the case of scattering spectroscopy, the intensity of electromagnetic radiation that has deviated from its original direction due to the presence of scattering centres in the substance is measured. The scattering can be elastic (e.g. Rayleigh) or inelastic (e.g. Raman).
The desire to understand and correctly identify the microscopic processes occurring within different materials has led to the continuous improvement of so-called time-resolved spectroscopy methods. These are based on the removal of a substance from a state of thermodynamic equilibrium by means of a well-defined optical pulse and the subsequent monitoring of the time course of the processes induced by this pulse. A typical example is the measurement of the lifetime of fluorescence or transient absorption.
Manufacturer:
- Edinburgh Instruments,
- Ocean Optics/Ocean Insight,
- Hamamatsu, Quantum Design Europe,
- Andor,
- Ultrafast Systems, NKT Photonics